Auditing Organization (AO) versus Notified Body (NB) versus Registrar. What's the difference? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog
Application for a Notified Body Opinion according to Article 117, Regulation (EU) 2017/745 on Medical Devices



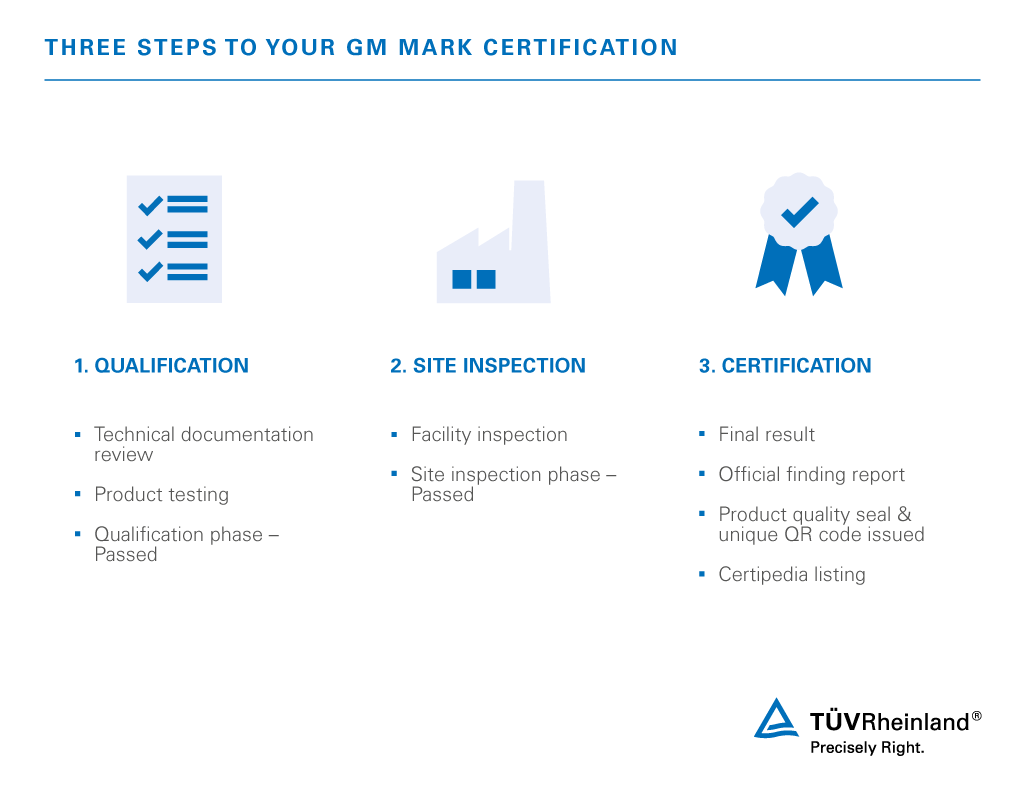
/tuv-rheinland-ivdr-visual-1-en_core_1_x.png)